
jdowning - 8-10-2010 at 06:31 AM
That culinary delight - meringue - is, on the face of it, a simple enough concoction of egg white and sugar. However, as no doubt as many a 'would be'
chef will admit, making meringue is not as easy as it might first appear.
The crucial part of the recipe is that of beating egg white with a whisk for several minutes until it forms a stiff stable foam after which sugar is
then added to 'fix' the foam prior to baking in an oven. There are many factors affecting successful formation of the foam including temperature,
humidity, surface tension of the egg white, type of mixing utensil, age of the egg, time of year the egg was laid, type of whisk or beater, duration
of whisking, bubble size etc. Above all the mixing vessel and whisk must be completely dry and totally free of any traces of oil or fat (such as egg
yolk or oils from fingers) that will prevent formation of the foam bubbles.
The foam is created due to the mechanical action of the whisk that alters the molecules of the albumen protein so that they solidify around air
bubbles also created by the whisking action.
For meringue, beating continues until the foam is very stiff but still slightly moist (when the mixing bowl is inverted the foam will not fall out).
At this stage, if the foam is left to stand a watery fluid will slowly accumulate at the bottom of the mixing bowl under the foam.
Whisking beyond this point causes the foam to become dry and hard - useless for cooking or any other purposes.
So - why all this talk about successfully making egg white foam? What does this have anything to do with preparing an egg white sealer or varnish?
Cennino d'Andrea Cennini wrote his little book "Il Libro dell' Arte' during the 15th C in Florence, Italy. It contains recipes, procedures and other
guidance that was of interest and practical value to painters, sculptors and other artists of his time. (A modern translation of the book is available
from Dover Publications, New York as "The Craftsman's Handbook").
In a chapter on varnishing Cennini describes how to quickly make carvings look as though they were varnished (but without having to use an oil
varnish).
To prepare the "varnish", the white of an egg is beaten as thoroughly as possible with a whisk so that it forms "a good, solid foam". The foam is then
left to "distil" overnight. The part that has distilled (i.e. the watery fluid in the bottom of the mixing bowl) is then applied to the work with a
"minerva" brush. (a minerva brush is a soft varnish brush made from the hair of an ermine or weasel). Cennini concludes this brief instruction by
commenting that the carvings of either wood or stone will look as though they were varnished and will be stronger.
(Cennini makes no mention of this treatment as being suitable for finishing lute sound boards - although he does recommend glue formulas for the
making and repair of lutes. This, of course, does not mean that egg white was never used as a sound board finish in earlier times).
Clearly the Cennini's initial step of first whisking the egg white to a solid foam and using only the distilled fluid had some significance - the
fluid perhaps having a different molecular structure than raw egg white.
So are those who today, in good faith, apply raw egg white to their sound boards missing a key step in preparation of the albumen?
Perhaps it would be a good idea to first spend some time in the kitchen making a few meringue pies - in order to learn how best to prepare and handle
egg white - before applying it to the sound board of an instrument.
FastForward - 8-10-2010 at 07:38 AM
Thanks John, this is fascinating. Like you said, the description is unlike what most people do when they apply the egg white to soundboards.
Anyway, I will probably keep the Meringue away from my oud. I will probably use a 1/2lb or 1lb cut of shellac to apply a very thin sealer coat on the
oud I am making.
SamirCanada - 8-10-2010 at 11:06 AM
Great find John!
Thanks for sharing.
jdowning - 8-10-2010 at 12:17 PM
I should add that - out of curiosity - I did a quick test using, for convenience, some supermarket egg white that happened to be in the 'fridge' -
just to get a feel for the possibilities (this stuff has likely been subject to pasteurisation so is not to be recommended in place of 'fresh' egg
albumen). The humidity levels are here quite high at present (an environment not recommended for beating egg white). Nevertheless, a fairly 'soft'
foam was the result and so the 'distillate' from this was brushed on to a test piece of Canadian 'silver spruce' soundboard wood using a soft Chinese
white goat bristle brush (too soft for applying an oil varnish). The watery consistency distillate fluid was immediately absorbed into the wood
(without any sign of brush marks) and appeared to dry quite quickly (within an hour) with minimal (if any) raising of the wood grain - although, not
surprisingly, some permanent darkening of the wood was apparent.
A further test using the white of a whole egg (Omega 3 - is this better than a good, old fashioned, free range 'farm' egg?) will be undertaken once
humidity levels drop to a more comfortable level.
Of course - historically -' the jury is still out' on whether or not some kind of 'invisible' sound board finish was ever applied to lutes or ouds.
jdowning - 8-12-2010 at 04:55 AM
Clearly your comments expressed here about turpentine have no relevance to this topic fernandraynaud.
If you want to experiment with and discuss the possibilities of mixing turpentine (or other oils) to egg white in an effort to make a sound board
finish other than one made from unadulterated egg white, then you should do so on one of the two current topics that you have already created on this
forum i.e. "Soundboard/face protection" or "Finishing the face with Egg White" - or create a new topic dealing specifically with your interest and
concerns in oil varnish components.
jdowning - 8-12-2010 at 12:11 PM
Thanks for removing your postings fernandraynaud.
Good luck with your alternative sound board finish experiments.
jdowning - 8-13-2010 at 05:12 AM
In an effort to try to understand what Cennini's egg white 'distillate' might consist of, some recent scientific work on egg white (albumin) foam
stability has been consulted. An understanding of the properties of albumin foams is of commercial interest to the food industry so the results of
much of the work done in this field is not freely accessible (and, in any case, is likely not readily understood by those of us who are not
microbiologists).
However, some papers reporting the results of experimental investigations into albumin foam stability do appear to provide some information that may
be of interest here. The stability of the foam in these investigations was assessed by measuring foam drainage volume and its chemical analysis (i.e.
Cennini's 'distillate') against beating time.
Raw egg white is made up of water and about 40 different proteins. The proteins each have a long chain molecular structure and in the unmodified
albumen just lie around curled up in a random mass - like a tangled ball of string. The proteins are of two types - those that are attracted to water
(hydrophilic) and those that are repelled by water (hydrophobic).
When raw egg white is whipped or beaten, the mechanical action of the beater causes the albumen to change from its natural state at a molecular level
(denaturation). The beating action also forms air bubbles. The denatured proteins unravel and bond together at the air/water interfaces forming a film
around the air bubbles to produce a foam.
The stability of an otherwise untreated albumin foam depends upon the level of denaturation of the protein molecules and bubble size (as well as the
presence of any foam destabilising agents such as fats or moisture). As beating time is increased, the air bubbles increase in number and decrease in
size creating a drier more stable foam. Excessive beating eventually causes the protein to coagulate into a hard foam devoid of fluid.
If a 'soft foam' (large air bubbles, lightly whipped) is left to stand, the foam will mostly break down into a watery 'distillate' like the original
egg white whereas a more stable stiff foam (formed by beating the egg white for several minutes) results in a much lower volume of 'distillate'.
Cennini instructs that the egg white be " beaten as thoroughly as possible so that it comes out a good solid foam" So it is the the properties of an
egg white foam beaten almost to the denaturation limit that is of interest here.
Let's now look at the experimental results reported by some researchers in this field of investigation.
SamirCanada - 8-13-2010 at 02:24 PM
Great research John,
thanks for contributing as I am anticipating to treat my next soundboard with this type of egg sealant formula.
Btw, will you be attending the Glengarry wood fair?
freya - 8-14-2010 at 04:54 AM
Just as a point of information, and the sample size is not large enough to be meaningful, the top of the one oud where I tried the egg-white finish
cracked a few weeks after the egg-white was applied. This was in the late Spring and the RH was back up in the 60s so I don't think dryness was an
issue - the top had made it through the previous Winter just fine. I would have dismissed this as a fluke but I spoke about this to a well-known oud
maker who said the same thing had happened to him and he would not use egg-white again as a result. I don't want to suggest any causality here, just
giving two, possibly meaningless, data points.
jdowning - 8-14-2010 at 08:16 AM
Thanks for that piece of information Harry. Was it raw egg white that caused the problem?
I shall eventually be testing the 'Cennini egg white foam distillate' on sound board test pieces in order to better understand the properties of the
distillate once dried so may be able to confirm if cracking is a potential problem.
So far I have not been able to verify if egg white raw or in any formulation was ever used as a sound board finish historically.
Humidity is still very high here at present so preparation of the 'distillate' will have to be postponed for the time being.
I will not be attending the Glengarry Wood Fair Samir as I am scheduled to present a workshop (talk/recital) at one of the other local museums on that
day (21 August at 2 pm).
The title of the workshop is "A Renaissance of the Lute" covering (briefly!) the development of the lute and its music in 16th C Europe.
The museum is the historic Macdonnel-Williamson House located at Pointe Fortune by the Chute-a-Blondeau - a rural setting near the Ontario/Quebec
border. Admission free.
http://www.mwhouse.ca/news_events.html
freya - 8-15-2010 at 05:00 AM
Hello John,
I just separated the yolk and the white and brushed the white onto the face - no heating or whipping. Though I think my wife used the yolk to make a
tasty coffee cake afterward...
jdowning - 8-15-2010 at 07:03 AM
Ah! One of the benefits of this line of investigation!
The food industry has a commercial interest in being able to manufacture baked goods of consistent quality. Research into the properties of egg white
foam in particular has, therefore, focussed in part on investigating how to produce the maximum volume of stable foam as well as assessing the food
value of the resultant foam. It has been found that the length of time that egg white is beaten or whisked is a critical factor. The volume of
drainage ("distillate") from egg white foam has also been used by investigators as an indicator in measuring foam volume, foam stability and the level
of denaturing of the foam.
For those who are interested in experimenting with egg white foam "distillate" as a sound board sealer here is a brief summary of the findings of
some of the food industry researchers - information that may be of some relevance.
- Egg white must be separated from a whole egg.
Commercially prepared egg whites do not foam well for two reasons - it is not possible to completely eliminate traces of egg yolk from commercially
prepared egg white (the fat in the yolk prevents foaming) and the egg whites are sterilised (pasteurised at 53 C)) with heat which causes partial
denaturation of the protein reducing foaming ability.
- All equipment used to prepare the foam must be completely free of traces of oil or grease. Do not use plastic bowls or utensils - plastic retains
oils and grease.
- moisture in the atmosphere has a negative effect on foam production. Do not beat egg whites when there is rain in the forecast or humidity levels
are high.
- egg white produces foam more quickly at room temperature rather than cooler refrigeration temperatures (surface tension is less at room
temperature).
- the optimum time of beating to produce a foam of maximum volume and stability is about 7 minutes (determined experimentally by measuring drainage
volume). Beating beyond this point results in extensive coagulation of the foam decreasing its water holding capacity and hence increasing drainage
volume. Beating for a shorter period of time produces a lower volume, coarser foam that is less stable allowing both unbeaten egg white and the foam
films to drain away as increased drainage.
- drainage from 'over beaten' foam is of lower viscosity (up to 50% lower) than that of the raw egg white (i.e. is more 'watery') and pH value
increases up to 9.3 (i.e. is more alkaline).
- the foam drainage contains diminished levels of protein (dependent upon beating time).
- The age of an egg is of no consequence when beating periods of over 4 minutes are involved.
Unfortunately the food industry researchers have no interest in the properties of foam drainage once it has dried out. That is for those of us
interested in the possibility of using the material as a wood sealer to discover by testing.
One question to resolve is for what period of time did Cennini beat his egg white to produce "a good solid foam"? Clearly the chemical composition and
hence properties of the "distillate" collected and used as a "varnish" for wood critically depends on this. Perhaps the first attempt to replicate
Cennini's instructions should be to beat the egg white for 7 minutes?
jdowning - 8-17-2010 at 12:36 PM
Question - is dried egg white "varnish" water resistant (if not fully waterproof)?
Henley's 20th C Book of Formulas, Processes and Trade Secrets describes an 'Albumen Paste' as follows :
"Fresh egg albumen is recommended as a paste for affixing labels on bottles. It is said that labels put on with this substance, and well dried at the
time, will not loosen even when bottles are put into water and left there for some time. Albumen, dry, is almost proof against molds or ferments."
So this might be confirmation that dried albumen is not only water resistant but resistant to decay. Testing required!
Cennini refers to the application of egg white 'distillate' on wood or stone (or a painting) as "How to make a Painting look as if it were Varnished.
In order to give one of your works the appearance of being varnished within a short time, without it actually being so ..."
An "appearance of being varnished" presumably means that - after an application (or several applications?) of the egg white 'distillate' - the surface
will be 'glossy' in appearance?
Cennini implies that the 'distillate' should be used as soon as it has formed 'overnight'. However, what if the 'distillate' was not used immediately
but left to concentrate (by evaporation) for several days before application? A sample of my supermarket egg whites has been left exposed to the
atmosphere now for over 10 days and is gradually drying up and becoming thicker and more viscous. This 'concentrated' material will now be applied to
a sound board test strip to check if it performs like a varnish and the remainder will be allowed to fully dessicate (dry out) to see if it can then
be dissolved in water - as a test of its water resistance.
It is interesting to note that the medical profession is using 'purified' (?) albumen to repair or seal holes or tears in membranes. One source
reports that even small holes in balloons may be successfully sealed with albumen. So, although when dried, albumen appears to be quite brittle, it
must have some residual flexibility and strength - not to mention resistance to fluid penetration (water included).
Interesting stuff!
fernandraynaud - 8-17-2010 at 02:44 PM
What I can contribute is that I am certain that my egg white is NOT waterproof. I applied 4 coats of plain unwhipped albumen, sanding
between coats. If the albumen builds up thicker, it polishes to a nice (but fragile) satin gloss. Or you can sand it down further and the spruce looks
almost untreated. I did one soundboard more glossy, and one more matte. The "dust" on the plastic steel wool has a white flaky appearance.
It happens that I literally drooled a drop on the more glossy soundboard, undoubtedly from a sense of awe. The saliva penetrated, dissolved in the
albumen; there was nothing to blot up. The next day it was difficult to buff it back to its previous state, as the top glossy layer had been reduced
on the area, presumably wicked into the wood. If it had been wine or coffee, I believe the water would have transported an ugly stain deep into the
wood. John, I would be surprised if your "distillate" somehow became water-resistant by evaporation. I can believe that albumen would make
labels adhere by occupying the gap and not "floating away" even if wet.
My question was simply what to use on soundboards. One value of egg-white coating is that it's a bit of a barrier to dirt; if nothing else
you can make a pass with fine steel wool and clean the soundboard that way, maybe reapply some egg white. It doesn't impart a yellowish hue. But
compared to shellac, which is both waterproof and resistant to lipids, I'd say it's vastly inferior as a soundboard coating. I'm very curious what you
will come up with.
jdowning - 8-17-2010 at 04:29 PM
I also am curious to find out if egg white might have properties that make it a suitable finish for sound boards - so will pursue this investigation a
bit further, with an open mind - in order to at least satisfy my curiosity. Note that I am not advocating egg white as a sound board finish and have
yet to find any evidence whatsoever that egg white was ever applied historically to either oud or lute sound boards (but oil varnishes or shellac
finishes certainly were not).
The vastly superior historical finish would, at the present time, appear to be - none!
I do not doubt that dried albumen is not completely waterproof like a varnish finish may be but I would expect the optimum foam distillate to have
some water resistance - yet to be confirmed by testing. In any case, what is supposed to be the virtue of a waterproof soundboard finish - apart from
providing some protection against gross abuse of an instrument?
BTW it is not 'my distillate' - I am simply trying to make sense of the historical information recorded by Cennini. Note that Cennini did not state
that this finish was suitable for lute sound boards either.
I became interested in this topic as a result of your question in another thread (that, for some unknown reason, promptly 'ground to a halt') which
was not simply 'what finish to use on sound boards' but more specifically "Has anyone applied egg white and then applied shellac/French polish"
The answer to that would seem to be 'yes'.
fernandraynaud - 8-18-2010 at 12:05 AM
Is everybody a little nervous today? I'm not in any way denigrating "your distillate" or your approach. I'm not accusing you of anything. I was just
passing on my accidental experiment specifically related to your topic, namely whether egg white can make a suitable coating for soundboards.
Personally, yes, I see value in a coating that resists soiling.
My question thread, that you apparently consider flawed enough to comment on, logically stopped when you very kindly answered my question, pointing me
towards people who use egg white as a sealer.
Though I don't understand what I've done to make you angry, I certainly didn't intend to.
jdowning - 8-18-2010 at 05:59 AM
I am not nervous or angry and was otherwise unware that you were accusing me of anything fernandraynaud.
BTW the topic of this thread is concerned with investigating the properties of the drainage (or distillate) from egg white foam - not those of raw egg
white - and the possible suitability of the distillate (not raw egg white) as a sound board sealer.
Researchers investigating properties of egg white foam and foam drainage have confirmed that at 'optimum' foam volume, the drainage collected contains
lower percentages of the protein content than raw egg white, is more alkaline and of lower viscosity. Also reported is that many of the denatured
protein structures have irreversibly changed characterised by the presence of sulphur atoms (sulfhydryl and disulphide) that apparently do not occur
in the raw egg albumin. Whether or not this will have any significant bearing on the properties of the distllate, once applied to wood and allowed to
dry, remains to be seen.
Those properties (hopefully) will be determined by, albeit low tech., experimental application.
The high humidity levels are starting to drop so there may be an opportunity soon to prepare the first experimental batch of egg white distillate for
testing.
jdowning - 8-22-2010 at 07:38 AM
There has been a brief drop in humidity levels so the opportunity was taken to prepare batch #1 of egg white foam distillate for testing.
Two large Omega 3 (low cholesterol yokes) were selected and the egg whites were separated from the yokes into a grease free bowl while still at
refrigerator temperature (easier to separate than at room temperature).
Unfortunately the yolk on the second egg broke and a small amount spilled into the separated egg white. This was removed with a clean spoon but as it
is not certain that every trace of yolk was removed the test will be repeated at a later date with another batch of egg white.
The egg whites were beaten for 7 minutes with an electric powered whisk running at half speed. This was sufficient to form a stiff foam which was
then left overnight. After 14 hours the foam had become lighter in texture (more like a detergent foam) and 15 ml of 'distillate' had collected in the
bottom of the bowl. This is batch #1
Conditions when egg white beaten - temp. 22 Celsius, 63% RH
Conditions after 14 hours - temp 20 Celsius, 69% RH.
The distillate was clear with slight yellow tint and viscosity like that of water. The alkalinity was measured at between pH 9 and pH 10 using litmus
test papers (this agrees with the findings of other researchers). The alkalinity of the raw egg white prior to beating was not tested.
A test piece of fine grained quarter sawn silver spruce, planed smooth, was then coated with the distillate - applied with a soft brush - until the
surface of the test piece was 'flooded'. The distillate was immediately absorbed by the test piece which was then left to dry for 24 hours.


jdowning - 8-22-2010 at 09:08 AM
The dried test piece surface was then 'polished' to a light sheen with a piece of fine cotton cloth.
To test the dried 'distillate' for fluid resistance, a drop of water was allowed to remain on the surface for 60 seconds, then blotted with a paper
towel and allowed to air dry. There was no obvious sign that the dried distillate had dissolved or allowed the water to penetrate the wood surface.
The test was repeated using a drop of red wine. Again none of the fluid was seen to have penetrated or stained the surface.
So from these two tests it would appear that a single coat of distillate - once dried - offers at least some level of water resistance and protection
from staining.
This should be sufficient time for a conscientious oud owner to immediately mop up any accidental fluid spills from a sound board to avoid any
residual marks or staining. Of course drinking and oud playing together is a practice not to be recommended!
Now to run a more extended test with water.


jdowning - 8-22-2010 at 09:37 AM
This time the drop of water was allowed to remain on the surface for over an hour (65 minutes to be exact) before being blotted and allowed to air
dry. Note that after an hour the water droplet has diminished in size but has not spread in area - indicating that some surface evaporation of the
water has taken place.
Under magnification (10X) a very faint white outline of part of the water droplet edge could be seen on the surface of the test piece. However, this
could be polished away with a cotton cloth becoming invisible - indicating that there perhaps may have been some slight dissolving of the 'distillate'
sealer. On the other hand, the water droplet was tap water that in this area is 'hard' containing calcium - so the 'white outline' may just be traces
of calcium deposits remaining after the water had evaporated and dried?
The test piece has now been given a second coat of 'distillate' over the first. The second coat appears to have been absorbed by the test piece and so
will be allowed to dry for 24 hours before repeating the droplet test.
Note the darkening of the surface of the test piece following application and drying of the 'distillate.


fernandraynaud - 8-22-2010 at 03:52 PM
This is really quite interesting. So something significantly changes at the molecular level as it sets, some sort of polymerization, since I assume
the solution is still mater-miscible. Is your wood as porous as typical soundboard?
Unseparated egg white behaves quite differently. I won't repeat your test with the wine, but you can see what happens to a layer of raw egg white.
Aqueous solutions penetrate and dissolve the polished surface. It's barely visible unless you look at an angle to the light. It's easy to fix by
applying some more, but of course it defeats the purpose, and any pigmented solution will have penetrated the wood. The virtue of plain egg white
coating is also its weakness: it's unobtrusive, easily touched up and easily removed. What has penetrated the wood during treatment is probably
entirely water, leaving the proteins as the fragile coating that buffs up so nicely.
Since you have the materials on hand, if you could try putting some unwhipped egg white on part of the other side of you board, let it dry or blow-dry
it, then see how the distillate interacts with it, it would be very helpful, as in light of your discovery that may be a better next step on these
soundboards than French polishing, for those of us who went with the plain egg white.

jdowning - 8-23-2010 at 12:11 PM
Your current experiences with raw egg white as a sound board finish/sealer are not relevant to this thread fernandraynaud!
Why not post them on either of your current topics "Soundboard/face protection" or "Finishing the face with Egg White" where they would not be out of
place?
fernandraynaud - 8-23-2010 at 01:56 PM
Please be nice, current scientific thinking is that making fellow-patients feel bad is not therapeutic.
In my view what I posted is on-topic enough. But if this is "your" thread, is this any way to treat guests? If you consider threads to be
private property, you logically should have stayed out of mine.

Returning to the thread, I was wondering if the distillate is still fully water-miscible, and if your test wood is as porous as typical soundboards.
And is there any chance you would be so kind as to try distillate on top of raw egg white?
jdowning - 8-24-2010 at 10:46 AM
Really?!
Regardless of what you want to believe fernandraynaud, the image that you posted of your oud with its failed raw egg white coating is not relevant to
this thread and neither is your question about any interaction there might be between dried raw egg white and foam 'distillate' although these might
not be out of place on your thread "Finishing the face with Egg White" that seems to have ground to a halt (?).
If you want to determine how egg white foam distillate reacts with the dried raw egg white coating on your ouds why don't you just go ahead and
prepare some 'distillate' as demonstrated earlier in this thread, run some tests yourself and report the results on your thread "Finishing the face
with Egg White"? You might then also end up with some first hand experience about preparing the distillate that could be of enough interest and
relevance to report on this thread.
When you ask "if the distillate is still fully water-miscible" I take it that you mean compared to raw egg white (both being in the liquid state)?
If I add water to the distillate a small quantity of a white sticky film forms on the surface - an indication perhaps that the distillate is not fully
water-miscible.
However, as Cennini makes no mention of diluting the distillate with water prior to its application then this is of little interest here.
Nevertheless, I am interested as to whether or not the dried residue of the distillate is soluble in water so currently have a small sample - set
aside two days ago - to air dry (slow).
On the question of the porosity of the wood sample, I previously reported that the first application of the distillate was immediately absorbed into
the wood.
The sample is very fine grained, quarter sawn 'Silver Spruce' set aside about 20 years ago as a promising looking sound board wood. 'Silver Spruce' is
a generic term used by the timber industry that might apply to any of the North American spruce varieties such as Sitka Spruce, Engelman Spruce,
Colorado Spruce etc.
For information and comparison the attached image shows the appearance of absorption of a water droplet on the untreated surface of the 'Silver
Spruce' sample as well as on a sample of 'Sitka Spruce' sound board material - after about a minute.

jdowning - 8-25-2010 at 05:16 AM
It is apparent from tests carried out so far that egg white foam distillate is quite different from raw egg white - although I do not pretend to
understand what has happened in the transformation at a molecular level or what the chemical analysis of the distillate might be.
I had eggs for breakfast so was able to test the pH level of the albumen before it was denatured by heat (i.e. cooked). An indicator strip showed a pH
value of 8 which agrees with values given in tables recording the pH values of common household materials (pH range for egg whites 7.6 to 8.0).
The pH scale is logarithmic (base 10) with neutral value (pure water) of 7.0.
So if the indicated pH value of 10 for the distillate that was measured the other day is representative, that means the distillate is 100 times more
alkaline than raw egg white.
When the distillate is applied to the test piece it is absorbed by the wood more quickly than water indicating that the distillate has a lower
viscosity (and surface tension?) than plain water.
Also the distillate which is quite transparent - when dried - permanently darkens the wood whereas plain water does not.
Cennini notes that his distillate not only gives work (wood, stone, paintings) the appearance of being varnished (darkened surface?) but also gives
the work added strength. This all suggests that there may be some kind of chemical reaction occurring between the distillate and the wood itself.
Tests carried out on hardwoods some time ago on this forum (see the 'marinated' wood topic) indicate that alkali solutions (such as diluted household
ammonia) react chemically with the wood cell structure as well as dissolving extractives within the cells (dependent upon the length of time wood is
immersed in the alkali solution). Softwoods were not tested as part of that investigation, however.
Household ammonia has a pH value of about 11.0 so is 10X more alkaline than the egg white foam distillate. To investigate if alkaline solutions do
react with softwood - perhaps partially dissolving the resins in the wood - a preliminary trial will be to 'varnish' the untreated surface of the test
piece with dilute ammonia, allow to dry and then compare the results with those already obtained for the distillate coating.
A further investigative step will then be to immerse a sample of softwood in the distillate for an extended period of time - just to see what
happens.
The sample of distillate that has already been set aside to dry into a solid residue (for solubility testing) will be regularly checked for pH value
as it (slowly) dries up and becomes more concentrated. So far there has been no detectable increase in alkalinity - although the pH test strips only
measure in unit steps so can only give an approximate indication of pH value.
fernandraynaud - 8-25-2010 at 03:54 PM
John, you had mentioned a while back you would test to determine if albumen is likely to cause cracks. How would you test for this, and are you
planning to do so?
jdowning - 8-25-2010 at 04:25 PM
Freya noted this as a problem encountered when using raw egg white as a sound board finish. I do not plan to test raw egg white on this thread - only
egg white foam distillate. However, if any of the test pieces should happen to crack during this investigation (doubtful in my opinion) then this will
be good reason to try to find the cause (which, of course, may have nothing whatsoever to do with albumen)
jdowning - 8-26-2010 at 05:26 AM
The test piece moistened with household ammonia solution has been allowed to dry overnight. The dried surface offered no resistance to the water
droplet test so the brief exposure of the wood to the alkaline solution (before evaporating) was not sufficient to significantly affect the wood cell
structure or dissolve the resins to form a water resistant coating.
The test piece with two coatings of distillate showed the same level of resistance as the single coating in the water droplet test. In this test a
water droplet was allowed to remain on the surface until fully evaporated (3 hours) and left a visible 'ring' or watermark that did not polish out
with a cloth. The attached macro image shows the 3hr residue next to the faint white residue left after a water droplet had remained on the surface
for 60 minutes, blotted and then allowed to dry.

jdowning - 8-29-2010 at 10:52 AM
A small sample (2.5 ml) of batch #1 of the egg white foam distillate had been set aside for a few days to completely evaporate.
The resultant residue remaining in the bottom of the measuring cup was clear and transparent with a very slight yellow tint. The dried residue was
brittle.
Water (2.5 ml) was then added to the residue which immediately started to dissolve (like a gum) becoming fully dissolved with stirring in about 5
minutes. The resultant fluid was clear and transparent with a slight yellow tint and tested at about pH 10 alkalinity.
The residue is therefore fully miscible in water and appears to have the same properties (?) as the original distillate when reconstituted.
The reconstituted distillate will again be left to fully evaporate - just to see what happens.

jdowning - 8-29-2010 at 12:25 PM
Another unknown factor that might be worth testing is that it is well known, in culinary circles, that beating egg whites in a copper bowl produces a
more stable foam (and presumably then has some effect on the quantity of the distillate and perhaps (?) its properties).
We do not know if Cennini used a copper bowl when beating his egg whites to a foam - but it is not beyond the bounds of possibility that he did.
fernandraynaud - 8-29-2010 at 03:25 PM
So, being water-soluble, how does this stuff seal the soundboard against water-borne stains?
jdowning - 8-30-2010 at 05:12 AM
As just demonstrated, when dried the distillate leaves behind a brittle, gum like residue that is water soluble. As it has also already been
demonstrated, when the distillate is applied to a wood surface and allowed to dry, the surface becomes water resistant. It might, therefore, be
concluded (as has already been suggested) that there is an interaction (physical and/or chemical) between the wood and distillate that creates a
barrier to moisture.
The distillate has low viscosity (and surface tension) and is quite alkaline so my guess is that - at a microscopic level - the distillate penetrates
deeply into the cell structure of the wood (drawn in by capillary action) where it then solidifies as a hard, brittle residue effectively plugging
(i.e. sealing) the cell cavities.
Due to its alkaline properties the distillate may also react with the cell walls of the wood perhaps to form a strong chemical bond with the wood and
become chemically altered in some way (less soluble perhaps?). As previously observed some change happens because the wood is darkened permanently by
the distillate and I know from past experiments that alkaline solutions do react chemically with wood cells and their contents eventually - on
prolonged immersion - causing the cells to permanently shrink and harden (see the 'Marinated Wood' topic on this forum).
Note that for a fluid to act as an effective sealer it does not have to be insoluble in water. For example thin glue size (a weak mixture of hide glue
in water) has been used for centuries to seal absorbent surfaces such as plastered walls. It would likely work just as well on wood surfaces.
(Note also that shellac/French polish is not proof against water damage).
Cennini makes no mention of his egg white distillate coated surfaces being waterproof or even water resistant - only that it gives work the appearance
of being 'varnished' (darkened?) as well as making the surface of the work stronger (and harder?).
It is interesting to note that some violin makers apply egg white distillate alone (or mixed with Gum Arabic and sugar - A.K.A. vernis bianca) to the
inside surfaces of their instruments claiming that this treatment significantly improves the acoustic response of even cheap, mass produced,
violins.
So, it may be that if egg white foam distillate is applied as a sound board sealer it not only affords some level of protection against moisture and
staining but may also improve the acoustic response of the instrument as well.
Of course if an oud with such a sound board finish is habitually handled by a player with dirty hands and clothes or while drinking and eating - not
to mention being left out in the rain - then a stained sound board (or worse) is likely inevitable.
jdowning - 8-31-2010 at 09:37 AM
This morning - after a period of 48 hours - the 'reconstituted' foam distillate residue, set aside to evaporate, had a slightly cloudy appearance due
to the presence of a lot of small particulate matter in suspension. So far there appears to be very little - if any - fluid lost due to
evaporation.
The fluid was examined under high magnification (X450) using a laboratory standard microscope. This revealed - apart from a few larger visible
particles of what seemed to be distillate residue (transparent, light yellow tinted) - a mass of microscopic particles of the same material
(transparent, light yellow tinted) in suspension, so small as to be invisible to the naked eye.
Hard to know what is going on here but it would seem that the addition of a relatively large volume of water to 'dissolve' the dried distillate
residue caused the residue - initially - to break up into minute fragments (molecular sized?) that then begin to rejoin together (by molecular
attraction?) over time into increasingly larger sized particles of the residue.
In order to test if the particles are joining together by some kind of molecular attraction and not as a result of fluid evaporation, water will be
added to the sample daily to compensate for any evaporation losses.
The microscope is an old but high optical quality Leitz compound microscope. It took a bit of adjustment to obtain the best light contrast to show up
the particles.
The attached images were taken with a Canon Powershot A470 digital camera, in 'point and shoot' mode, simply held over the eyepiece of the microscope
- hence the 'vignetting'. All a bit 'rough and ready' but it worked well enough to give a reasonable appreciation of what could be seen under the
microscope.
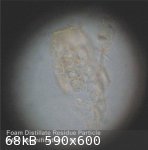

jdowning - 9-6-2010 at 04:44 PM
After a long period of hot, humid conditions, the weather has moderated sufficiently to allow preparation of batch #2 of egg white foam distillate.
Separation of egg white from the yolk is a delicate enough operation in order to avoid breaking the yolk and contaminating the albumen. The best way
is to tap the egg shell (in the centre) on a hard surface just sufficient to crack the shell. The shell halves are then separated sufficiently to
allow the albumen to drain into a clean, grease free mixing bowl.
The albumen of two large sized eggs was then beaten to a stiff foam with an electric whisk - set at half speed - for 7 minutes. The foam was then left
overnight (for 12 hours). Conditions at start were temperature 26 Celsius, Relative Humidity 57% and at finish 21 Celsius, 63% Relative Humidity. The
distillate collected measured 50 ml - clear with light yellow tint.
The remaining foam was quite firm and stiff.
The pH value of the raw albumen was about 8.0 and that of the distillate collected about 10.0.
jdowning - 9-8-2010 at 06:04 AM
With sufficient quantity of batch #2 distillate available samples of silver spruce have been prepared and left to soak in the distillate in a sealed
container. This test is to find out if there might be a reaction between the alkaline distillate and the wood.
After 24 hours immersion of the wood samples, the distillate had darkened in colour from the pale lemon of the distillate to a light brown tint that
appeared to be slightly cloudy. Small bubbles of gas (air?) could be seen coming from the wood samples forming larger, stable bubbles on the surface
of the distillate.
Microscopic examination showed that the cloudiness is due to many solid particles in suspension. The particles have a random shape and lemon yellow
tint looking very much like the distillate residue particles in the microscopic images previously posted.
This morning (three days later) the surface bubbles have reduced in size and quantity and emmission of bubbles from the samples seems to have ceased.
This may indicate complete saturation of the samples with the didtillate.
The distillate is now full of particles - visible to the naked eye - giving the distillate a clouded appearance.


jdowning - 9-8-2010 at 06:32 AM
Here are the images missing from the previous posting


jdowning - 9-8-2010 at 11:58 AM
The wood sample will be left soaking in the distillate in order to see how the wood is affected by the alkalinity of the distillate.
As a further test - for purposes of comparison - samples of silver spruce and sitka spruce have been allowed to soak in household ammonia solution in
sealed containers. Household ammonia is a 5% solution of ammonia in water with a pH value of about 11. The greater alkalinity should, therefore,
accelerate the test into a shorter time period than for the distillate.
After soaking for 24 hours in the clear ammonia solution the solution in the silver spruce container had changed to a clear wine red colour and that
of the sitka spruce to a clear pale yellow colour. Clearly some kind of chemical reaction is in progress.


jdowning - 9-11-2010 at 03:55 PM
After 6 days immersion in Distillate #2, the silver spruce sample seems to be fully saturated. The distillate is 'muddy' in appearance due to the
presence of many particles in suspension.
Under the microscope - in addition to larger particles of what seem to be distillate residue there are a number of 'tree like' growths (dendrites)
where individual cells seem to be joining together into longer chains.
Not sure what to make of this at present - so just for the record and information.


jdowning - 9-11-2010 at 04:33 PM
Batch #2 foam distillate is being tested as a coating on a sample of quarter cut Sitka Spruce sound board wood. The distillate was brushed on so that
the surface of the sample was 'flooded' and then allowed to dry for 24 hours. The distillate caused the wood to darken in colour and a distinct
distillate residue accumulation was noted at the edge of the coated surface.
The coated surface was then tested for water resistance by allowing a large water droplet (11 mm diameter) to remain on the surface until completely
evaporated (several hours). This left a ring of distillate residue at the perimeter of the droplet. The area inside the ring was paler in colour than
the distillate coating outside
So it would appear that the water 'dissolved' the insoluble component of the dried distillate into smaller residue particles which then migrated
(under surface tension?) to the outer edges of the water droplet.


jdowning - 9-12-2010 at 04:46 AM
The area within the ring of distillate residue appears to have been cleared of residue on the surface of the wood sample. Is this area water resistant
due to a chemical reaction and/or molecular bonding between the distillate and the wood cells?
To answer this question a droplet of water was placed in the centre of the ring and allowed to remain for 15 minutes. It can be seen from the shape of
the droplet that it is sitting upon an impervious surface (compare this to a water droplet on a waterproof plastic surface). After 15 minutes the
droplet was removed by blotting with an absorbent paper towel. After all moisture had dried there was no visible evidence of any water staining by the
droplet or absorption of moisture into the wood.
This confirms that the surface is at least water resistant due to the presence of distillate that has soaked into in the wood cells. So it would
appear that after flooding the surface of the wood some distillate is absorbed into the wood cells sealing them against moisture absorption while the
surplus distillate dries out on the surface of the wood as a 'varnish' layer that can be 'dissolved' by the addition of water.


jdowning - 9-12-2010 at 05:07 AM
The next test was to 'wash' the dried surface of the test piece - coated with distillate - with water. Water was brushed over an area of the test
piece (measuring 30 mm X 60 mm) - flooding the surface - and then allowed to dry for 24 hours.
Even with an area of this size distillate residue was observed to accumulate around the edges of the 'washed' area.
Two water droplets were placed on the surface - a small droplet measuring about 4 mm in diameter and a larger drop measuring about 12 mm across.
After 90 minutes the small droplet was blotted with absorbent paper. There was no visible evidence of water stain or absorption by the wood.
The large drop was allowed to remain on the surface to partially evaporate for 7 hours before being blotted and dried. There was no visible evidence
of the water being absorbed by the wood although there were some slight traces of distillate residue that had formed around the perimeter of the drop
- presumably due to traces of distillate residue remaining on the surface after 'washing'.
Enlarging the macro images of the large drop revealed what appeared to be minute bubbles of a gas on the surface of the wood - presumably similar to
the gas bubbles observed during the immersion test previously posted. Sign perhaps of a chemical reaction taking place within the wood cells?
The next trial will be to coat a larger test piece of Sitka Spruce with distillate followed by a water coating and buffing to remove any dried
distillate residue remaining on the surface. Then to run the water droplet test again



jdowning - 9-16-2010 at 11:40 AM
After soaking in foam distillate for 7 days, the silver spruce samples have been removed and allowed to dry. The colour of the distillate has changed
from a transparent pale lemon tint to a cloudy orange.
For information, bubbles initially forming on the surface of the distillate had remained stable for several days but eventually disappeared after 7
days. It is interesting to note that stable bubbles formed again on shaking the jar containing the distillate.
The microscopic 'tree like' dendritic cell formations previously reported may have appeared as a consequence of the break down of the bubbles - the
dendrites being part of the molecular 'network' structure surrounding the air bubbles and giving the bubbles (foam) their stability. So it would seem
that the property of the beaten and denatured egg white molecules to form a stable foam remains - to some extent - in the foam distillate.
No dimensional change is obvious in the dried test samples although there has been a distinct darkening in colour of the samples (as well as the
distillate). This indicates that there has been some kind of reaction between the alkaline distillate and the wood cells.
Microscopic examination of the end grain of the wood samples was inconclusive in that did not reveal any obvious visible change in cell structure or
dimensions.
Test samples of silver spruce and sitka spruce immersed in an even more alkaline 5% Ammonia solution for 10 days and allowed to dry showed no obvious
dimensional change but both samples and ammonia solutions also had darkened in colour.
This completes the immersion testing.
Next to report on tests with larger samples of Sitka Spruce sound board material coated with egg white foam distillate.



jdowning - 9-16-2010 at 12:15 PM
A larger sample of Sitka Spruce, cut 'on the quarter' and scraped smooth, measures about 10 cm wide by 15 cm long by 2 mm thick.
This has been coated with batch #2 foam distillate brushed on like a varnish and allowed to dry for 24 hours followed by clean water brushed on and
allowed to dry for 24 hours.
Note that the batch #2 distillate for the test has been kept in a sealed container at room temperature for 12 days with no sign of deterioration.
The immediate effect of coating the sample with distillate is that the sample distorts (bows) due to the expansion of the wood cells as they quickly
absorb the distillate. The distortion is significant - about 4 mm over 10 cm. On a sound board, say, 30 cm wide this distortion would amount to 12
mm.
After drying for 24 hours the sample lost its distorted curve and became flat again.
Therefore, for anyone contemplating using egg white foam distillate as a sound board sealer this is something to be taken into account. An oud sound
board is prevented from expanding across its width by the braces glued in place. So any expansion of the wood cells caused by absorption of the
distillated must be temporarily accommodated by compression of the moist wood cell walls until all water has evaporated. If this is done slowly over
24 hours or so (i.e. in relatively high humidity conditions, say 70% RH +), then, perhaps, no permanent damage will result. However, if the drying is
rapid (low humidity conditions, say 40% RH or so) then it is possible that shrinkage cracking may be a destructive consequence in a braced sound
board.
Egg white foam distillate is not the same stuff as raw egg white but the cracking of sound boards coated with raw egg white - as previously reported
by freya - may be due to a similar mechanism?

jdowning - 9-19-2010 at 12:36 PM
After the larger test piece had been coated with #2 distillate and allowed to dry a second coating of plain water again caused the test piece to
temporarily distort into a curve before drying flat again after 24 hours.
The large water droplet test was then repeated. After 15 minutes a droplet (about 12 mm across) was blotted with a paper towel. Once any surface
moisture had evaporated there was no visible evidence of the droplet remaining.
A droplet remaining on the surface for 3 hours was again blotted and allowed to dry. The area covered by the droplet became slightly white after
drying.
After 8 hours, a water droplet was reduced in size (due to evaporation) but showed no signs of absorption into the wood surface.
After 24 hours the water droplet had completely evaporated leaving - the now familiar - ring of distillate residue defining the perimeter of the water
droplet.
So, coating the Sitka Spruce sound board sample with egg white foam distillate caused a temporary distortion (bowing) of the sample. After drying for
24 hours the sample became flat again.
Then, after applying a brush coat of plain water, the sample became temporarily distorted but, after drying for 24 hours, the sample again became flat
again.
Interesting in that the distillate coated sample does not allow penetration of water, even after 24 hours exposure, yet the distillate residue coating
the surface of the wood sample - after all water content has evaporated - is itself readily 'soluble' in water.
To follow - I shall try to draw some kind of conclusion from these tests!



jdowning - 9-20-2010 at 12:32 PM
It is interesting to note that the bubbles or foam generated by shaking the #2 distillate (left after the immersion tests - previously reported) is
still remains after 4 days - although somewhat reduced in quantity. This confirms that the egg white foam distillate retains the ability to form a
relatively stable foam - the denatured, long chain protein molecules linking together in a network around air bubbles.
How might this interesting property of the distillate account for the apparent water resistance of a sound board material coated with distillate when
the distillate residue - when dried - is 'soluble' in water?
Time to speculate.


jdowning - 9-24-2010 at 10:16 AM
For comparison, the Sitka spruce wood samples were tested in order to determine if the egg white foam distillate is as effective a sealer for varnish
as it is for water.
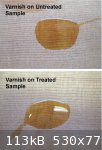
For the first test a drop of relatively 'thick' (viscous) oil varnish was applied to an untreated wood sample and to one coated with distillate. After
24 hours there was little evidence of any absorption of the varnish in either case - i.e. the varnish remained as a surface coating. The drops when
dried measured about 3 cm. across in each case
A second test was to apply a drop of 'thin' (low viscosity) varnish (Minwax Antique Oil) to the wood samples. The oil finish quickly spread (in a
matter of minutes) across the surface of both samples to cover an area measuring about
7 cm across in each case after the oil had dried. On examination, the oil had been fully absorbed into the wood on the untreated sample (leaving a
dull 'matt' finish) but remained on the surface of the treated sample (glossy, 'varnished finish).

Estebanana _Stephen Faulk - 9-26-2010 at 02:30 PM
There is name for this substance it's called "glair" any egg white preparation used as finish or sealer is glair.
Cennini may or may not have mentioned in his book it was usually used to seal wood panels before painting with egg tempera. I've made it and used it
on instruments as a sealer under French polish. It's actually quite unecesessary and only turns the wood darker and raises the soft grain. It's great
if you want an instrument to have an antique lumpy look before using a regular finish like varnish or shellac.
On a historical note Cennini was one of the great narcissists of his day. When he was walking across a grassy area one morning with a companion he
pointed to his shadow and said see the rainbow halo around my head this proves I am a genius. It was the halation of the sun light shining through
morning dew, which of course has the same effect on everyones shadow. His book was also compiled of information which was common knowledge at the time
if you worked in a guild shop as an artisan, Cennini merely had enough money to edit the information and compile it to sell as a book.
IF anyone has ever thrown an egg on your car you know how difficult it is to get it off after it has set a few days. It's the proteins in the yolk
which make it last an be so annoyingly difficult if not impossible to remove. Well sir, that's is why egg tempera painting lasts 500 years and glair
finishes ( egg white ) does not.
But Cennini failed to mention that because he was to busy attending to his beautifully coiffed hair do in order to attract the attentions of wanton
females so he could disperse yet another type of protein.
jdowning - 9-27-2010 at 08:35 AM
The problem with the term 'glair' is that it can apply to either raw egg white or to Cennini's egg white foam 'distillate' - so I have chosen
'distillate' to distinguish it from raw egg white. As this investigation has shown, egg white foam distillate is not the same stuff as raw egg
white.
The ambiguous terminology seems have resulted in some confusion among those violin makers in particular who choose to use 'glair' or its variants
(such as vernis bianca) as a sealer or ground applied to the inside surfaces of their instruments - some claiming that this treatment results in
significantly improved acoustical performance. So a luthier using this stuff might apply a 'glair' of raw egg white or egg white foam distillate or
either mixed with other 'improving' additions such as gum Arabic, honey, molasses etc. based upon myth, or what they may have read somewhere of
recommendations handed down (and possibly misremembered in the course of time) from luthiers past.
The 19th C Spanish luthier Torres is said to have applied raw egg white to his guitar sound boards - or was it egg white foam distillate .....?
The purpose of this investigation has been simply to get a better understanding of the properties of egg white foam distillate so that others might
have a better idea of what to expect if they decide to try it as a sealer for oud or lute sound boards. No recommendations for this application either
way though.
As I do not plan to proceed further with any more tests I shall conclude with a summary of the properties of egg white foam distillate as determined
in the course of this investigation.
Estebanana _Stephen Faulk - 9-27-2010 at 02:36 PM
Just put some on an oud and let someone sweat on it for a year. Then you'll know what it does.
jdowning - 9-27-2010 at 03:42 PM
Really?!
Estebanana _Stephen Faulk - 9-27-2010 at 04:06 PM
Doh!
jdowning - 9-28-2010 at 06:25 AM
Before moving on to summarise the properties of egg white foam distillate a short test was carried out on the sound board of my old Egyptian oud
(currently undergoing restoration) to try to establish if any sort of invisible finish was present. The sound board shows no signs of localised
staining but has a darkish uniform patina. Initial thoughts were that a thin wax finish had been applied (as it had been to the bowl) - but could it
have been egg white distillate - or just no finish at all?
The attached images show the difference between a water drop applied to untreated Sitka Spruce compared to one applied to a waxed sample of Sitka
Spruce (Minwax Paste Finishing Wax). As might be expected the water on the untreated sample remained on the surface for about 30 minutes being
immediately but slowly and completely absorbed into the wood whereas that on the waxed surface showed no sign of being absorbed even after an hour or
so.
In comparison, the water droplet on the old Egyptian oud sound board showed the same characteristics as the untreated Sitka Spruce sample.
Had the sound board been treated with egg white foam distillate then the water would have remained on the surface until fully evaporated as is evident
in the previously posted images of the test samples.
Wiping the sound board surface with a damp cloth removed some oxidation and surface grime.
It is, therefore, concluded that the sound board originally had no finish of any kind - which is consistent with traditional oud making practice.
Some lute makers (e.g. the late Robert Lundberg) apply a thin hard wax finish to their sound boards which would certainly seal the surface against
water absorption. There is no evidence that I am aware of, however, to confirm that the old lute sound boards, like those of the oud, were ever
finished or sealed in any way.
Apparently, the old oud makers (working in the Turkish tradition at least) were very fastidious in preventing anyone but themselves from touching
their soundboards during the final stages of assembly - for fear of accidental (or deliberate!) contamination of the wood with oils that they felt had
a deleterious effect on the sound board response.


jdowning - 10-2-2010 at 12:34 PM
To tie up the loose ends of this thread in summary:
Egg white foam distillate is not the same stuff as raw egg white. Raw egg white is clear and gelatinous whereas the distillate is a uniform,
transparent pale yellow liquid of low viscosity (watery consistency). It is considerably more alkaline than raw egg white.
According to food industry researchers, albumen is made up of a complex mix of protein molecules some that are attracted to water and some that are
repelled by water. Mechanical agitation of the albumen causes the protein molecules to denature - to open out into long chains that link together to
form a membrane or 'skin' around included air bubbles to create the stable 'egg white foam' of commercial interest to the food industry. The
researchers also report that the denatured molecules in the foam have been irreversibly modified characterised by the presence of sulphur atoms
(sulfhydryl and disulphide).
The volume of foam generated is dependant upon the time it is beaten (trial indicate that about 7 minutes is optimum). Stability of the foam is
adversely affected by the presence of moisture or contamination by traces of oil or grease - so equipment must be clean and the egg white beaten when
humidity is low.
When the foam is left to stand, the foam begins to break down as the molecules gradually lose their adhesion to each other (presumably because of
moisture in the air). The resultant distillate that drains from the foam after 14 hours or so presumably is a mixture of denatured water attracting
and water repelling molecules in a solution of water (as agitating the liquid distillate results in the formation of bubbles that are stable - lasting
for days).
When the distillate is allowed to dry out, by evaporation of the water content, a clear, brittle, gum like residue remains. This residue is readily
'dissolved' in water.
When the distillate is brushed onto sound board material it is immediately absorbed into the wood causing (temporary) swelling of the wood cells. When
dry, the surface treated with distillate exhibits resistance to water absorption. It is thought that the reason for this is that the distillate
residue absorbed by the wood is partially reconstituted by moisture. The molecules then immediately link together around any air trapped in the cell
cavities to form a stable foam preventing further absorption of moisture.
The alkaline distillate also has a chemical reaction with the wood perhaps resulting in a close bonding with the cell structure?
So wood treated with distillate should be a good sealer against moisture ingress which is perhaps why some violin makers report improved acoustic
response when the interior (not exterior) surfaces of their instruments are coated with distillate (or its variants) - the coating sealing the wood
against atmospheric humidity changes that can temporarily affect acoustic performance? Some even claim that cheap Chinese violins show dramatic
improvement when treated this way.
It has been demonstrated by these tests that the distillate can also act as a sealer for varnishes although when using oil varnishes it is usual
practice to first 'prime' the wood with thin, diluted varnish (or shellack). In the case of shellack, it is generally well known that shellack used by
itself is a very effective sealer of wood grain.
So - there you have it - in a nutshell:
If the top surface of an oud sound board is to be left unvarnished and otherwise untreated in the traditional way then there would obviously be little
point in sealing only the underside with egg white foam distillate to prevent atmospheric moisture ingress in order to gain some - as yet unproven -
acoustical stability or other advantage. However, if the sound board top surface is to be non traditionally varnished or shellacked then a sealing
coat of distillate on the underside just might show some benefit.
For those willing to experiment - let us all know the results. Good luck!
Greg - 10-3-2010 at 03:01 AM
Thank you John. This has been an extremely interesting series and I sincerely thank you for your diligence and for sharing the results of this
important research with us.
Warm regards,
Greg
antekboodzik - 3-24-2015 at 09:23 AM
Dear Sir John,
Did your trials confirm if using egg white distillate makes wood surface stronger, as mentioned by Cennini?
I am asking, because I wonder, if this sealer would be good for lute neck and pegbox, making its surfaces more resistant to make dents on it whentying
frets, or sometimes scratches of nail of the left hand thumb...
Is it also safe to use it on quite delicate, renaissance lute bowl? Wouldn't it distort the bowl?
jdowning - 3-24-2015 at 11:27 AM
I doubt if application of the distillate would significantly affect surface strength of wood to be of practical use in preventing damage such as
denting, scratching etc.
Can't see that it would cause distortion of a lute bowl either.

