| Pages:
1
2
3 |
fernandraynaud
Oud Junkie
    
Posts: 1865
Registered: 7-25-2009
Location: San Francisco, California
Member Is Offline
Mood: m'Oudy
|
|
John, you had mentioned a while back you would test to determine if albumen is likely to cause cracks. How would you test for this, and are you
planning to do so?
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
Freya noted this as a problem encountered when using raw egg white as a sound board finish. I do not plan to test raw egg white on this thread - only
egg white foam distillate. However, if any of the test pieces should happen to crack during this investigation (doubtful in my opinion) then this will
be good reason to try to find the cause (which, of course, may have nothing whatsoever to do with albumen)
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
The test piece moistened with household ammonia solution has been allowed to dry overnight. The dried surface offered no resistance to the water
droplet test so the brief exposure of the wood to the alkaline solution (before evaporating) was not sufficient to significantly affect the wood cell
structure or dissolve the resins to form a water resistant coating.
The test piece with two coatings of distillate showed the same level of resistance as the single coating in the water droplet test. In this test a
water droplet was allowed to remain on the surface until fully evaporated (3 hours) and left a visible 'ring' or watermark that did not polish out
with a cloth. The attached macro image shows the 3hr residue next to the faint white residue left after a water droplet had remained on the surface
for 60 minutes, blotted and then allowed to dry.

|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
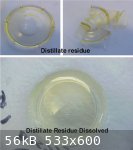
A small sample (2.5 ml) of batch #1 of the egg white foam distillate had been set aside for a few days to completely evaporate.
The resultant residue remaining in the bottom of the measuring cup was clear and transparent with a very slight yellow tint. The dried residue was
brittle.
Water (2.5 ml) was then added to the residue which immediately started to dissolve (like a gum) becoming fully dissolved with stirring in about 5
minutes. The resultant fluid was clear and transparent with a slight yellow tint and tested at about pH 10 alkalinity.
The residue is therefore fully miscible in water and appears to have the same properties (?) as the original distillate when reconstituted.
The reconstituted distillate will again be left to fully evaporate - just to see what happens.

|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
Another unknown factor that might be worth testing is that it is well known, in culinary circles, that beating egg whites in a copper bowl produces a
more stable foam (and presumably then has some effect on the quantity of the distillate and perhaps (?) its properties).
We do not know if Cennini used a copper bowl when beating his egg whites to a foam - but it is not beyond the bounds of possibility that he did.
|
|
|
fernandraynaud
Oud Junkie
    
Posts: 1865
Registered: 7-25-2009
Location: San Francisco, California
Member Is Offline
Mood: m'Oudy
|
|
So, being water-soluble, how does this stuff seal the soundboard against water-borne stains?
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
As just demonstrated, when dried the distillate leaves behind a brittle, gum like residue that is water soluble. As it has also already been
demonstrated, when the distillate is applied to a wood surface and allowed to dry, the surface becomes water resistant. It might, therefore, be
concluded (as has already been suggested) that there is an interaction (physical and/or chemical) between the wood and distillate that creates a
barrier to moisture.
The distillate has low viscosity (and surface tension) and is quite alkaline so my guess is that - at a microscopic level - the distillate penetrates
deeply into the cell structure of the wood (drawn in by capillary action) where it then solidifies as a hard, brittle residue effectively plugging
(i.e. sealing) the cell cavities.
Due to its alkaline properties the distillate may also react with the cell walls of the wood perhaps to form a strong chemical bond with the wood and
become chemically altered in some way (less soluble perhaps?). As previously observed some change happens because the wood is darkened permanently by
the distillate and I know from past experiments that alkaline solutions do react chemically with wood cells and their contents eventually - on
prolonged immersion - causing the cells to permanently shrink and harden (see the 'Marinated Wood' topic on this forum).
Note that for a fluid to act as an effective sealer it does not have to be insoluble in water. For example thin glue size (a weak mixture of hide glue
in water) has been used for centuries to seal absorbent surfaces such as plastered walls. It would likely work just as well on wood surfaces.
(Note also that shellac/French polish is not proof against water damage).
Cennini makes no mention of his egg white distillate coated surfaces being waterproof or even water resistant - only that it gives work the appearance
of being 'varnished' (darkened?) as well as making the surface of the work stronger (and harder?).
It is interesting to note that some violin makers apply egg white distillate alone (or mixed with Gum Arabic and sugar - A.K.A. vernis bianca) to the
inside surfaces of their instruments claiming that this treatment significantly improves the acoustic response of even cheap, mass produced,
violins.
So, it may be that if egg white foam distillate is applied as a sound board sealer it not only affords some level of protection against moisture and
staining but may also improve the acoustic response of the instrument as well.
Of course if an oud with such a sound board finish is habitually handled by a player with dirty hands and clothes or while drinking and eating - not
to mention being left out in the rain - then a stained sound board (or worse) is likely inevitable.
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
This morning - after a period of 48 hours - the 'reconstituted' foam distillate residue, set aside to evaporate, had a slightly cloudy appearance due
to the presence of a lot of small particulate matter in suspension. So far there appears to be very little - if any - fluid lost due to
evaporation.
The fluid was examined under high magnification (X450) using a laboratory standard microscope. This revealed - apart from a few larger visible
particles of what seemed to be distillate residue (transparent, light yellow tinted) - a mass of microscopic particles of the same material
(transparent, light yellow tinted) in suspension, so small as to be invisible to the naked eye.
Hard to know what is going on here but it would seem that the addition of a relatively large volume of water to 'dissolve' the dried distillate
residue caused the residue - initially - to break up into minute fragments (molecular sized?) that then begin to rejoin together (by molecular
attraction?) over time into increasingly larger sized particles of the residue.
In order to test if the particles are joining together by some kind of molecular attraction and not as a result of fluid evaporation, water will be
added to the sample daily to compensate for any evaporation losses.
The microscope is an old but high optical quality Leitz compound microscope. It took a bit of adjustment to obtain the best light contrast to show up
the particles.
The attached images were taken with a Canon Powershot A470 digital camera, in 'point and shoot' mode, simply held over the eyepiece of the microscope
- hence the 'vignetting'. All a bit 'rough and ready' but it worked well enough to give a reasonable appreciation of what could be seen under the
microscope.
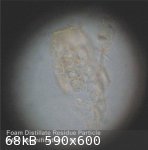

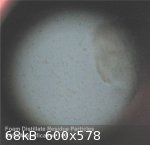
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
After a long period of hot, humid conditions, the weather has moderated sufficiently to allow preparation of batch #2 of egg white foam distillate.
Separation of egg white from the yolk is a delicate enough operation in order to avoid breaking the yolk and contaminating the albumen. The best way
is to tap the egg shell (in the centre) on a hard surface just sufficient to crack the shell. The shell halves are then separated sufficiently to
allow the albumen to drain into a clean, grease free mixing bowl.
The albumen of two large sized eggs was then beaten to a stiff foam with an electric whisk - set at half speed - for 7 minutes. The foam was then left
overnight (for 12 hours). Conditions at start were temperature 26 Celsius, Relative Humidity 57% and at finish 21 Celsius, 63% Relative Humidity. The
distillate collected measured 50 ml - clear with light yellow tint.
The remaining foam was quite firm and stiff.
The pH value of the raw albumen was about 8.0 and that of the distillate collected about 10.0.
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
With sufficient quantity of batch #2 distillate available samples of silver spruce have been prepared and left to soak in the distillate in a sealed
container. This test is to find out if there might be a reaction between the alkaline distillate and the wood.
After 24 hours immersion of the wood samples, the distillate had darkened in colour from the pale lemon of the distillate to a light brown tint that
appeared to be slightly cloudy. Small bubbles of gas (air?) could be seen coming from the wood samples forming larger, stable bubbles on the surface
of the distillate.
Microscopic examination showed that the cloudiness is due to many solid particles in suspension. The particles have a random shape and lemon yellow
tint looking very much like the distillate residue particles in the microscopic images previously posted.
This morning (three days later) the surface bubbles have reduced in size and quantity and emmission of bubbles from the samples seems to have ceased.
This may indicate complete saturation of the samples with the didtillate.
The distillate is now full of particles - visible to the naked eye - giving the distillate a clouded appearance.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
Here are the images missing from the previous posting
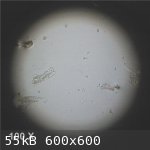


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
The wood sample will be left soaking in the distillate in order to see how the wood is affected by the alkalinity of the distillate.
As a further test - for purposes of comparison - samples of silver spruce and sitka spruce have been allowed to soak in household ammonia solution in
sealed containers. Household ammonia is a 5% solution of ammonia in water with a pH value of about 11. The greater alkalinity should, therefore,
accelerate the test into a shorter time period than for the distillate.
After soaking for 24 hours in the clear ammonia solution the solution in the silver spruce container had changed to a clear wine red colour and that
of the sitka spruce to a clear pale yellow colour. Clearly some kind of chemical reaction is in progress.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
After 6 days immersion in Distillate #2, the silver spruce sample seems to be fully saturated. The distillate is 'muddy' in appearance due to the
presence of many particles in suspension.
Under the microscope - in addition to larger particles of what seem to be distillate residue there are a number of 'tree like' growths (dendrites)
where individual cells seem to be joining together into longer chains.
Not sure what to make of this at present - so just for the record and information.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
Batch #2 foam distillate is being tested as a coating on a sample of quarter cut Sitka Spruce sound board wood. The distillate was brushed on so that
the surface of the sample was 'flooded' and then allowed to dry for 24 hours. The distillate caused the wood to darken in colour and a distinct
distillate residue accumulation was noted at the edge of the coated surface.
The coated surface was then tested for water resistance by allowing a large water droplet (11 mm diameter) to remain on the surface until completely
evaporated (several hours). This left a ring of distillate residue at the perimeter of the droplet. The area inside the ring was paler in colour than
the distillate coating outside
So it would appear that the water 'dissolved' the insoluble component of the dried distillate into smaller residue particles which then migrated
(under surface tension?) to the outer edges of the water droplet.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
The area within the ring of distillate residue appears to have been cleared of residue on the surface of the wood sample. Is this area water resistant
due to a chemical reaction and/or molecular bonding between the distillate and the wood cells?
To answer this question a droplet of water was placed in the centre of the ring and allowed to remain for 15 minutes. It can be seen from the shape of
the droplet that it is sitting upon an impervious surface (compare this to a water droplet on a waterproof plastic surface). After 15 minutes the
droplet was removed by blotting with an absorbent paper towel. After all moisture had dried there was no visible evidence of any water staining by the
droplet or absorption of moisture into the wood.
This confirms that the surface is at least water resistant due to the presence of distillate that has soaked into in the wood cells. So it would
appear that after flooding the surface of the wood some distillate is absorbed into the wood cells sealing them against moisture absorption while the
surplus distillate dries out on the surface of the wood as a 'varnish' layer that can be 'dissolved' by the addition of water.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
The next test was to 'wash' the dried surface of the test piece - coated with distillate - with water. Water was brushed over an area of the test
piece (measuring 30 mm X 60 mm) - flooding the surface - and then allowed to dry for 24 hours.
Even with an area of this size distillate residue was observed to accumulate around the edges of the 'washed' area.
Two water droplets were placed on the surface - a small droplet measuring about 4 mm in diameter and a larger drop measuring about 12 mm across.
After 90 minutes the small droplet was blotted with absorbent paper. There was no visible evidence of water stain or absorption by the wood.
The large drop was allowed to remain on the surface to partially evaporate for 7 hours before being blotted and dried. There was no visible evidence
of the water being absorbed by the wood although there were some slight traces of distillate residue that had formed around the perimeter of the drop
- presumably due to traces of distillate residue remaining on the surface after 'washing'.
Enlarging the macro images of the large drop revealed what appeared to be minute bubbles of a gas on the surface of the wood - presumably similar to
the gas bubbles observed during the immersion test previously posted. Sign perhaps of a chemical reaction taking place within the wood cells?
The next trial will be to coat a larger test piece of Sitka Spruce with distillate followed by a water coating and buffing to remove any dried
distillate residue remaining on the surface. Then to run the water droplet test again



|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
After soaking in foam distillate for 7 days, the silver spruce samples have been removed and allowed to dry. The colour of the distillate has changed
from a transparent pale lemon tint to a cloudy orange.
For information, bubbles initially forming on the surface of the distillate had remained stable for several days but eventually disappeared after 7
days. It is interesting to note that stable bubbles formed again on shaking the jar containing the distillate.
The microscopic 'tree like' dendritic cell formations previously reported may have appeared as a consequence of the break down of the bubbles - the
dendrites being part of the molecular 'network' structure surrounding the air bubbles and giving the bubbles (foam) their stability. So it would seem
that the property of the beaten and denatured egg white molecules to form a stable foam remains - to some extent - in the foam distillate.
No dimensional change is obvious in the dried test samples although there has been a distinct darkening in colour of the samples (as well as the
distillate). This indicates that there has been some kind of reaction between the alkaline distillate and the wood cells.
Microscopic examination of the end grain of the wood samples was inconclusive in that did not reveal any obvious visible change in cell structure or
dimensions.
Test samples of silver spruce and sitka spruce immersed in an even more alkaline 5% Ammonia solution for 10 days and allowed to dry showed no obvious
dimensional change but both samples and ammonia solutions also had darkened in colour.
This completes the immersion testing.
Next to report on tests with larger samples of Sitka Spruce sound board material coated with egg white foam distillate.



|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
A larger sample of Sitka Spruce, cut 'on the quarter' and scraped smooth, measures about 10 cm wide by 15 cm long by 2 mm thick.
This has been coated with batch #2 foam distillate brushed on like a varnish and allowed to dry for 24 hours followed by clean water brushed on and
allowed to dry for 24 hours.
Note that the batch #2 distillate for the test has been kept in a sealed container at room temperature for 12 days with no sign of deterioration.
The immediate effect of coating the sample with distillate is that the sample distorts (bows) due to the expansion of the wood cells as they quickly
absorb the distillate. The distortion is significant - about 4 mm over 10 cm. On a sound board, say, 30 cm wide this distortion would amount to 12
mm.
After drying for 24 hours the sample lost its distorted curve and became flat again.
Therefore, for anyone contemplating using egg white foam distillate as a sound board sealer this is something to be taken into account. An oud sound
board is prevented from expanding across its width by the braces glued in place. So any expansion of the wood cells caused by absorption of the
distillated must be temporarily accommodated by compression of the moist wood cell walls until all water has evaporated. If this is done slowly over
24 hours or so (i.e. in relatively high humidity conditions, say 70% RH +), then, perhaps, no permanent damage will result. However, if the drying is
rapid (low humidity conditions, say 40% RH or so) then it is possible that shrinkage cracking may be a destructive consequence in a braced sound
board.
Egg white foam distillate is not the same stuff as raw egg white but the cracking of sound boards coated with raw egg white - as previously reported
by freya - may be due to a similar mechanism?

|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
After the larger test piece had been coated with #2 distillate and allowed to dry a second coating of plain water again caused the test piece to
temporarily distort into a curve before drying flat again after 24 hours.
The large water droplet test was then repeated. After 15 minutes a droplet (about 12 mm across) was blotted with a paper towel. Once any surface
moisture had evaporated there was no visible evidence of the droplet remaining.
A droplet remaining on the surface for 3 hours was again blotted and allowed to dry. The area covered by the droplet became slightly white after
drying.
After 8 hours, a water droplet was reduced in size (due to evaporation) but showed no signs of absorption into the wood surface.
After 24 hours the water droplet had completely evaporated leaving - the now familiar - ring of distillate residue defining the perimeter of the water
droplet.
So, coating the Sitka Spruce sound board sample with egg white foam distillate caused a temporary distortion (bowing) of the sample. After drying for
24 hours the sample became flat again.
Then, after applying a brush coat of plain water, the sample became temporarily distorted but, after drying for 24 hours, the sample again became flat
again.
Interesting in that the distillate coated sample does not allow penetration of water, even after 24 hours exposure, yet the distillate residue coating
the surface of the wood sample - after all water content has evaporated - is itself readily 'soluble' in water.
To follow - I shall try to draw some kind of conclusion from these tests!



|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
It is interesting to note that the bubbles or foam generated by shaking the #2 distillate (left after the immersion tests - previously reported) is
still remains after 4 days - although somewhat reduced in quantity. This confirms that the egg white foam distillate retains the ability to form a
relatively stable foam - the denatured, long chain protein molecules linking together in a network around air bubbles.
How might this interesting property of the distillate account for the apparent water resistance of a sound board material coated with distillate when
the distillate residue - when dried - is 'soluble' in water?
Time to speculate.


|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
For comparison, the Sitka spruce wood samples were tested in order to determine if the egg white foam distillate is as effective a sealer for varnish
as it is for water.
For the first test a drop of relatively 'thick' (viscous) oil varnish was applied to an untreated wood sample and to one coated with distillate. After
24 hours there was little evidence of any absorption of the varnish in either case - i.e. the varnish remained as a surface coating. The drops when
dried measured about 3 cm. across in each case
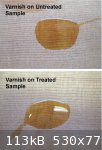
A second test was to apply a drop of 'thin' (low viscosity) varnish (Minwax Antique Oil) to the wood samples. The oil finish quickly spread (in a
matter of minutes) across the surface of both samples to cover an area measuring about
7 cm across in each case after the oil had dried. On examination, the oil had been fully absorbed into the wood on the untreated sample (leaving a
dull 'matt' finish) but remained on the surface of the treated sample (glossy, 'varnished finish).

|
|
|
Estebanana _Stephen Faulk
Oud Addict
  
Posts: 25
Registered: 6-16-2010
Member Is Offline
|
|
There is name for this substance it's called "glair" any egg white preparation used as finish or sealer is glair.
Cennini may or may not have mentioned in his book it was usually used to seal wood panels before painting with egg tempera. I've made it and used it
on instruments as a sealer under French polish. It's actually quite unecesessary and only turns the wood darker and raises the soft grain. It's great
if you want an instrument to have an antique lumpy look before using a regular finish like varnish or shellac.
On a historical note Cennini was one of the great narcissists of his day. When he was walking across a grassy area one morning with a companion he
pointed to his shadow and said see the rainbow halo around my head this proves I am a genius. It was the halation of the sun light shining through
morning dew, which of course has the same effect on everyones shadow. His book was also compiled of information which was common knowledge at the time
if you worked in a guild shop as an artisan, Cennini merely had enough money to edit the information and compile it to sell as a book.
IF anyone has ever thrown an egg on your car you know how difficult it is to get it off after it has set a few days. It's the proteins in the yolk
which make it last an be so annoyingly difficult if not impossible to remove. Well sir, that's is why egg tempera painting lasts 500 years and glair
finishes ( egg white ) does not.
But Cennini failed to mention that because he was to busy attending to his beautifully coiffed hair do in order to attract the attentions of wanton
females so he could disperse yet another type of protein.
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
The problem with the term 'glair' is that it can apply to either raw egg white or to Cennini's egg white foam 'distillate' - so I have chosen
'distillate' to distinguish it from raw egg white. As this investigation has shown, egg white foam distillate is not the same stuff as raw egg
white.
The ambiguous terminology seems have resulted in some confusion among those violin makers in particular who choose to use 'glair' or its variants
(such as vernis bianca) as a sealer or ground applied to the inside surfaces of their instruments - some claiming that this treatment results in
significantly improved acoustical performance. So a luthier using this stuff might apply a 'glair' of raw egg white or egg white foam distillate or
either mixed with other 'improving' additions such as gum Arabic, honey, molasses etc. based upon myth, or what they may have read somewhere of
recommendations handed down (and possibly misremembered in the course of time) from luthiers past.
The 19th C Spanish luthier Torres is said to have applied raw egg white to his guitar sound boards - or was it egg white foam distillate .....?
The purpose of this investigation has been simply to get a better understanding of the properties of egg white foam distillate so that others might
have a better idea of what to expect if they decide to try it as a sealer for oud or lute sound boards. No recommendations for this application either
way though.
As I do not plan to proceed further with any more tests I shall conclude with a summary of the properties of egg white foam distillate as determined
in the course of this investigation.
|
|
|
Estebanana _Stephen Faulk
Oud Addict
  
Posts: 25
Registered: 6-16-2010
Member Is Offline
|
|
Just put some on an oud and let someone sweat on it for a year. Then you'll know what it does.
|
|
|
jdowning
Oud Junkie
    
Posts: 3485
Registered: 8-2-2006
Location: Ontario, Canada
Member Is Offline
Mood: No Mood
|
|
Really?!
|
|
|
| Pages:
1
2
3 |